



Fundamentals of Medical Oncology
Carcinogenesis: the biology of neoplasia and the medical treatment of cancer
The transformation of a normal cell into a malignant cell involves a series of mutations in genes, termed oncogenes, that directly contribute to neoplasia when their functions are altered.
Oncogenes normally function in cellular processes such as cell division, apoptosis, and differentiation.
Examples of oncogenes:
Growth factor receptors
Tyrosine kinases play essential roles in transmitting mitogenic signaling pathways.
Genes that regulate the cell division cycle
Genes that regulate programmed cell death programmed
Genes involved in DNA repair
Tumour genesis is a multistep process that requires the accumulation of multiple mutations.
All tumours share a number of attributes that contribute to their malignant phenotype, but the specific molecular events that produce these phenotypes vary greatly among tumor types and individuals.
Carcinogenesis is a multistep process, and the development of fully malignant cancers requires many independent events.
It is known that these steps involve the acquisition of mutations in cancer genes and that genetic instability allows these rare events to accumulate at a rate that ultimately gives rise to aggressive neoplasms.
Although the specific mutations that cause human cancers vary greatly between types of cancers and individuals, the broad consequences of these mutations are abnormal phenotypes that are shared by most cancers.
The Hallmarks of Cancer
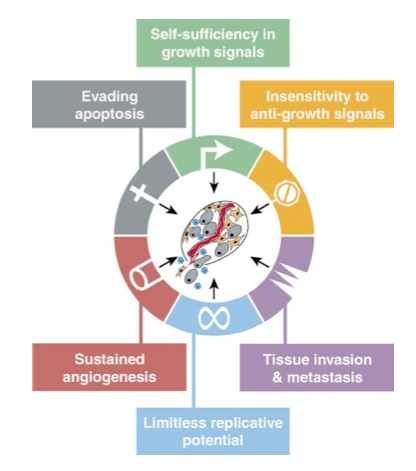
Weinberg and Hanahan in 2000[1] proposed six essential capabilities found in tumour cells that encompass the “hallmarks of cancer.”
self-sufficiency in growth signals
insensitivity to antigrowth signals
evasion of apoptosis
limitless replicative potential
sustained angiogenesis
tissue invasion and metastasis
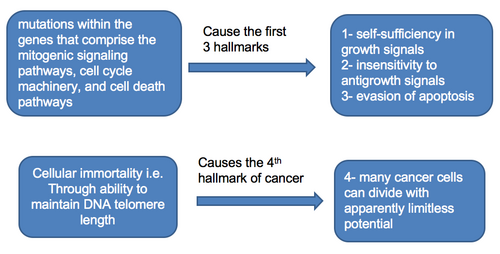
Hallmarks 1 to 4
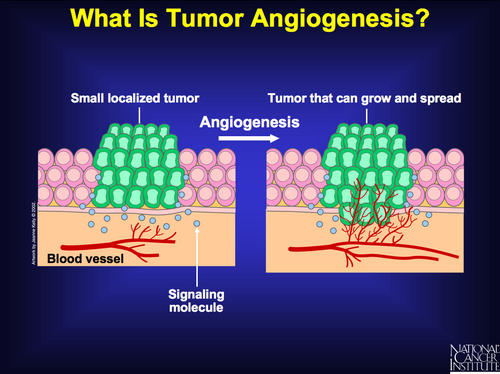
Sustained Angiogensis: the 5th hallmark of cancer
The capability of sustained angiogenesis reflects the fact that tumours often outgrow their blood supply and must actively recruit vasculature in order to grow.
In normal tissues, the development of new blood vessels is highly regulated by both positive and negative signals.
Tumor cells promote angiogenesis by upregulating the pathways that promote blood vessel formation (e.g., increased expression of growth factors such as vascular endothelial and fibroblast growth factors) and by reducing the activity of inhibitory pathways.
Metastases and invasion: the 6th and most unique of the hallmarks of cancer
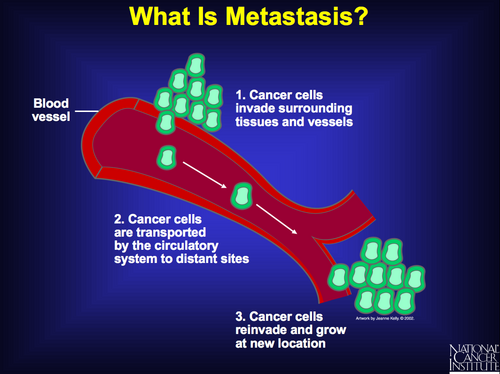
The last of these six capabilities, tissue invasion and metastasis, is the most heterogeneous and poorly understood. It is, however, critically important because metastasis accounts for most cancer fatalities.
Distant metastases are tumour cells that have broken away from the primary tumour, have traveled to other parts of the body, and have begun to grow at the new location.
Distant stage is also called remote, diffuse, disseminated, metastatic, or secondary disease. In most cases there is not a continuous trail of tumour cells between the primary site and the distant site.
Tissue invasion is extension from the primary organ beyond adjacent tissue into the next organ; for example, from the lung through the pleura into bone or nerve.
Mechanisms of Metastasis
1- Lymph channel spread
Travel in lymph channels beyond the first (regional) drainage area to lymph nodes that are considered remote from the primary site.
Tumor cells can be filtered, trapped and begin to grow in any lymph nodes in the body
2- Hematogenous or blood-borne metastases
Invasion of blood vessels within the primary tumor allows escape of tumor cells or tumor emboli which are transported through the blood stream to another part of the body where it lodges in a capillary or arteriole.
At that point the tumor penetrates the vessel wall and grows into the surrounding tissue
3-Spread through fluids in a body cavity
Example: malignant cells rupture the surface of the primary tumor and are released into the thoracic or peritoneal cavity.
They float in the fluid and begin to grow on any tissue reached by the fluid. This type of spread is also called implantation or seeding metastases.
Metastases and invasion: role of genes
It is becoming clear that specific gene products are associated with the ability of tumor cells to metastasize to different organ sites.
Elegant animal metastasis models, as well as transcriptional profiling of human cancers, are revealing that metastasis includes alterations in genes involved in processes such as cell adhesion, integrin signaling, growth factors, chemokine signal transduction, and extracellular proteolysis
Tumour Growth
Skipper’s Laws
These laws are based on work on a mouse leukemia cell line and indicate that all cells are cycling, none are in resting phase, and will proliferate exponentially, doubling at a fixed rate. This gives a false impression of accelerated growth over time.
the doubling time of proliferating cells is constant
drug induced kill follows first-order kinetics
for a given tumour, the percentage of cells killed at a given drug and dose is unrelated to tumour burden and is constant
fractional cell kill depends on inherent sensitivity to agent and dose of agent, he extent of cell kill is a stable percentage and not related to number of cells in tumour
This would suggest that cell kill would increase with repeated treatment and increased dose.
Problems with this theory:
model applies to proliferating cells
solid tumours have varying proportions of proliferating cells (vary by tumour type and over time within same tumour, balance between cell loss and doubling time of the tumour, key intrinsic tumour characteristics that are important for cytotoxic treatment effects such as growth fraction and cell cycle/phase duration)
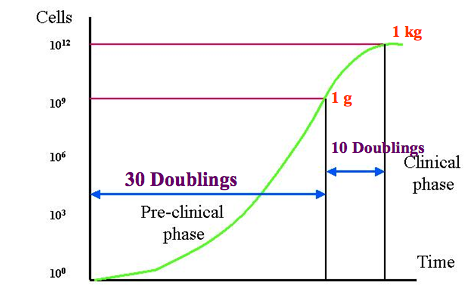
threshold of clinical detection (1 cm = 1g = 1billion cells)
lethal tumour burden approximately 1 kg = 1 trillion cells - there is only a 3 log difference between earliest clinically detectable and lethal tumour burden
Gompertzian model
In this model the tumour growth over time follows a sigmoid curve. As the tumour grows, the doubling time increases and the growth fraction decreases. Even though early growth is exponential and becomes slower over time cells accumulate slowly at first when few are dividing. The maximum growth rate is at approximately 1/3 of maximal tumour volume and slows to a plateau near lethal tumour volume. This is consistent with the sense that tumours appear to grow more quickly once detected.
Goldie-Coldman Hypothesis
At any given time within a solid tumour, a proportion of cells are inherently drug-resistant, the proportion of these cells increases with tumour size.
Limits of our knowledge
heterogeneity of cancer (growth rate/cell cycle kinetics, biochemical characteristics determining drug sensitivity)
extrapolation to human scenario from lab and animal science
_________________________________
1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
 Previous
Previous