



Cytotoxics
Discovery of cytotoxic agents has been primarily empirical; based on observation and experience. The screening of natural products, antibiotics and chemical synthetics and testing in in vitro and in vivo tumour model systems including human tumour cell lines has led to the approval and availability of > 50 cytotoxic agents.
Cytotoxics target DNA (direct toxicity, interference with replication and synthesis) and the process of cell division. Many cytotoxics share mechanisms of action.
CLASS | DRUG | ACTION |
antimetabolites | 5FU, cytarabine, purine analogs, methotrexate | mainly active during synthetic phase of cell cycle, structural analogs of normal metabolite that are incorporated into DNA/RNA causing false message transmission enzyme inhibitors for the synthesis of essential compounds |
mitotic inhibitors | vincas, taxanes | mainly active during mitotic phase, vincas bind to tubulin causing metaphase arrest, taxanes enhance microtubule assembly forming a stable nonfunctional microtubule |
alkylating agents | cyclophosphamide, cisplatin, carmustine | DNA cross linking inhibiting DNA replication and RNA transcription |
antitumour antibiotics | anthracyclines, bleomycin | intercalating agents, insertion between DNA base pairs |
topoisomerase inhibitors | camptothecins, anthracyclines, etoposide | inhibit enzymes that break and reseal DNA strands |
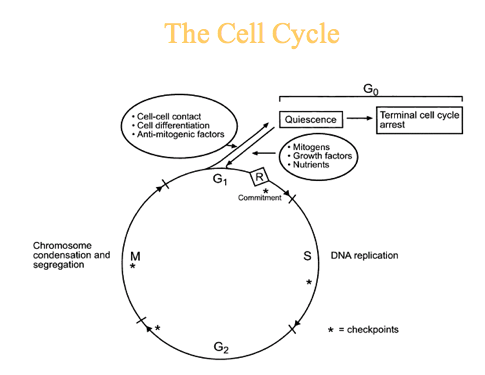
Sites of Action
| G1 | S | G2 | M |
Alkylators | x | x | x | x |
Platinums | x | x | x | x |
Tumour antibodies |
| x* |
|
|
Antimetabolites |
| x |
|
|
Vinca alkaloids |
|
|
| x |
Taxanes |
| x |
| x |
Principles
Theory points to need to give repeated doses of cytotoxic agents. The optimal timing of repeat doses for individual drug depends on whether it is cell cycle phase specific and the normal tissue toxicity of drug and its recovery period.
Cell cycle specific agents (in S or M phase) are more active when give frequently or continuously. This allows new cells not yet in cycle to enter into vulnerable state. So, repeated smaller doses are preferable to fewer larger doses.
All models of tumour growth support that antineoplastic drugs be administered:
in combination
in an alternating fashion
at the maximally tolerated dose
over the shortest time possible
Dose, dose intensity and schedule are important contributors to outcome in oncology.
The dose intensity is the drug dose per unit of time.
Drugs are given in doses and schedules to balance the toxic effects and efficacy. Efficacy is more dependent on inherent sensitivity of the tumour to a particular agent than on how the agent is given.
Most gains are to be made from combination treatment. Single agent treatment has limited value except in rare cases.
Ideal combinations of drugs are those which have:
activity in the same tumour type
different mechanisms of action (and resistance)
non-overlapping toxicity (though this is not possible with full doses of each drug)
acceptable side effects
Toxicity occurs acutely and in the long term.
ACUTE TOXICITY | AGENT |
nausea and vomiting |
|
mucositis |
|
alopecia |
|
myleosuppression | almost all |
local complications of extravasation |
|
hepatic toxicity | methotrexate |
pancreatitis | arabinoside |
cystitis | cyclophosphamide, ifosfamide |
neurotoxicity | cerebellar toxicity with arabinoside |
CHRONIC TOXICITY |
|
second malignancies | alkylating agents, anthracyclines |
cardiac | anthracyclines, cyclophosphamide |
myelosuppression | alkylators |
pulmonary | bleomycin, cyclophosphamide |
renal | cisplatinum |
ototoxicity | cisplatinum |
gonadal (sterility) | alkylators |
neurologic | vincas, taxanes, platinum, thalidomide |
Toxicity Prevention:
prophylactic antiemetics
vascular access devices minimize extravasation
adequate pre/post hydration
stop below known toxic cumulative doses
dose reduction / delay
growth factor support
prophylactic antibiotics
mouth care
cytoprotectants / rescue agents
Maintain a high index of suspicion and intervene early!
Limits of cytotoxics
only 3-4 drug combination regimens are possible
DNA synthesis/replication as a target is limited because of normal tissue toxicity
 Previous
Previous